-
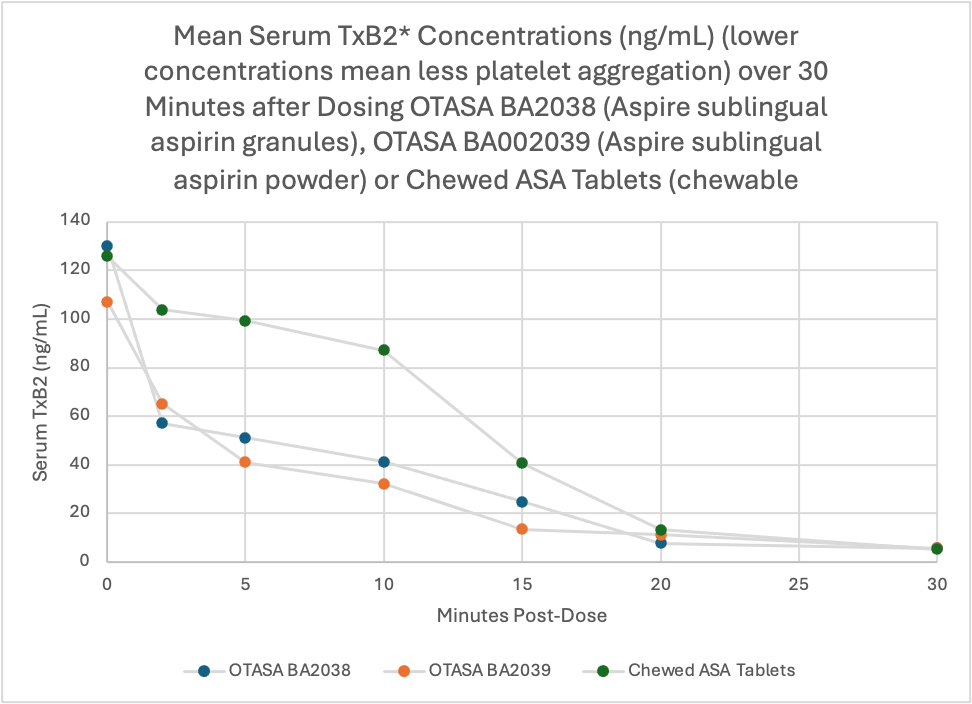
Aspire’s sublingual aspirin formulation significantly inhibited serum thromboxane B2 (TxB2) within the first two minutes after dosing
-
TxB2 is a biomarker indicating aspirin’s effect on platelet aggregation, the clumping of platelets that leads to dangerous blood clots
-
This rapid inhibition is critical during suspected heart attacks, when clots blocking heart arteries can cause permanent muscle damage
-
Lab results showed Aspire’s product acted approximately twice as fast as the current recommendation for chewed aspirin tablet treatment
-
These clinical trial results enable a potential regulatory submission for accelerated approval on track for Q3 2025
ESTERO, FLORIDA / ACCESS Newswire / September 3, 2025 / Aspire Biopharma Holdings, Inc. (NASDAQ:ASBP) (“Aspire” or the “Company”), developer of a multi-faceted patent-pending drug delivery technology, today announced positive final results from its randomized, crossover bioavailability trial to assess the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of Aspire’s investigational new sublingual aspirin product compared to chewed uncoated aspirin tablets in healthy adults. The final results demonstrated that Aspire’s sublingual aspirin product produced higher and more rapid mean plasma concentrations of acetylsalicylic acid (ASA, the active antiplatelet form of aspirin) compared to chewed aspirin tablets (p0.05), as detailed in Aspire’s previous press release. The product was also safe and well-tolerated by patients, and no adverse events were reported. However, even more clinically significant results were discovered since the press release announcing top-line results was issued on August 18, 2025.
Important New Findings
Aspire’s OTASA BA2039 sublingual formulation also significantly inhibited serum thromboxane B2 (TxB2) within the first two minutes after dosing compared to the chewed oral aspirin tablets (p0.02). TxB2 is a biomarker for the effect of aspirin on platelet aggregation. This has significant and important positive implications for its role in the inhibition of blood clotting and its role in heart attacks.
These important results underscore the potential of Aspire’s sublingual aspirin product to be the market-leading treatment for suspected acute myocardial infarction (AMI, blockage of blood flow to heart muscle causing damage or death of heart tissue – commonly known as a “heart attack”). There are an estimated 18 million Americans living with coronary artery disease with approximately 800,000 per year experiencing an AMI leading to 300,000 deaths.
According to a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines dated April 1, 2025, “Aspirin has long been considered an integral part of anti-platelet therapy to prevent recurrent atherothrombotic events among patients with Acute Coronary Syndromes (ACS). Aspirin reduces the incidence of vascular death after AMI, and in secondary prevention trials (that include patients after MI), it re-duces the occurrence of vascular and coronary events, including MI and stroke.
Aspirin should be initiated with a loading dose (162-325 mg) in patients with ACS without an absolute contraindication as soon as possible on presentation irrespective of final management strategy (invasive or noninvasive).” Aspire’s sublingual product rapidly delivers 162.5 mg of aspirin, precisely in the range suggested by this study, and Aspire’s product begins to show TxB2 inhibition in two minutes or less.”
Kraig Higginson, Interim Chief Executive Officer of Aspire, commented, “The final results from our recent clinical trial confirm our belief that our high dose sublingual aspirin has to the potential to be a life-changing, market-leading treatment for patients experiencing a suspected heart attack. Patients in our study experienced a significantly improved time of inhibition of TxB2, a biomarker for platelet aggregation, the process where activated platelets stick to each other to block the flow of blood to heart muscle. We believe our new sublingually delivered aspirin has the potential to save lives. We look forward to presenting these data to the FDA as we move down the development pathway towards potential commercialization of our safe, novel and effective sublingual form of high dose aspirin. We hope the day will soon come that every EMT, emergency service provider, and hospital will have our sublingual aspirin on hand for those suffering from heart attacks.”
* TxB2 is a stable metabolite of thromboxane A2 (TxA2), a potent chemical that promotes platelet clumping and vasoconstriction. Measuring low levels of TxB2 confirms that aspirin is working as intended.
The results of Aspire’s clinical study suggest that sublingual administration of aspirin provides earlier drug exposure and platelet inhibition than the current standard-of-care, which is chewing and swallowing uncoated aspirin tablets. Numerous published studies indicate that an even more rapid pharmacodynamic effect would be desirable during the early phase of thrombus evolution in the setting of myocardial infarction or ischemic stroke (i.e., the sooner TxB2 concentration can be reduced, the better for the patient). Reducing mean serum TxB2 concentration after aspirin dosing-which the clinical study demonstrates that Aspire’s sublingual aspirin product does rapidly, starting in 2 minutes or less–indicates successful inhibition of platelet aggregation, which may reduce the risk of vascular death and other major adverse cardiovascular events.
Advancing Aspire’s Sublingual Aspirin
Aspire plans to review its clinical trial results with the U.S. Food and Drug Administration (FDA) to enable a potential regulatory submission for accelerated approval in the third quarter of 2025.
About Aspire’s Clinical trial AB-101
Clinical trial AB-101 was a randomized crossover bioavailability study of Aspire’s investigational new sublingual aspirin product compared to chewed uncoated aspirin tablets. Six otherwise healthy 40 to 65-year-old subjects were administered 162 mg aspirin as a single dose in each of three treatment periods separated by two 14-day washout periods. Two different investigational sublingual powder and granule formulations (Aspire Biopharma) and chewed uncoated oral aspirin tablets (Bayer) were studied. The primary objective of the clinical trial was to evaluate the bioavailability of ASA in plasma over eight hours after dosing. An additional objective was to evaluate the potentially life-saving effect of each treatment on serum TxB2.
Aspire’s sublingual aspirin is an investigational new drug and has not been approved for marketing by FDA or any other government regulatory authority.
About Oral Aspirin
Oral aspirin is FDA-approved for treatment of suspected AMI with the initial dose of 160-162.5 mg is administered as soon as an AMI is suspected.i In a large, multicenter study of aspirin, streptokinase, and the combination of aspirin and streptokinase in 17,187 patients with suspected AMI, aspirin treatment produced a 23 percent reduction in the risk of death from cardiovascular diseases within five weeks.ii Clinical practice guidelines recommend that aspirin be initiated as soon as possible with the initial dose chewed, when possible, to achieve faster onset of antiplatelet action.iii
About Aspire Biopharma Holdings, Inc.
Aspire Biopharma has developed a patent-pending sublingual delivery technology that can deliver drugs to the body rapidly and precisely. This technology offers the potential to improve effectiveness and reduce side effects by going directly to the bloodstream and avoiding the gastrointestinal tract. Aspire Biopharma’s delivery technology can be applied to many different active pharmaceutical ingredients (APIs) and other bioactive substances, spanning both small and large molecule therapeutics, nutraceuticals and supplements.
For more information, please visit www.aspirebiolabs.com
Safe Harbor Statement
This press release contains “forward-looking statements” within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended, which are intended to be covered by the “safe harbor” provisions created by those laws. Aspire’s forward-looking statements include, but are not limited to, statements regarding our or our management team’s expectations, hopes, beliefs, intentions or strategies regarding our future operations. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,” “believe,” “contemplate,” “continue,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “will,” “would,” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking. These forward-looking statements represent our views as of the date of this press release and involve a number of judgments, risks and uncertainties. We anticipate that subsequent events and developments will cause our views to change. We undertake no obligation to update forward-looking statements to reflect events or circumstances after the date they were made, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. Accordingly, forward-looking statements should not be relied upon as representing our views as of any subsequent date. As a result of a number of known and unknown risks and uncertainties, our actual results or performance may be materially different from those expressed or implied by these forward-looking statements. Some factors that could cause actual results to differ include general market conditions, whether clinical trials demonstrate the efficacy and safety of our drug candidates to the satisfaction of regulatory authorities, or do not otherwise produce positive results which may cause us to incur additional costs or experience delays in completing, or ultimately be unable to complete the development and commercialization of our drug candidates; the clinical results for our drug candidates, which may not support further development or marketing approval; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials and marketing approval; our ability to achieve commercial success for our drug candidates, if approved; our limited operating history and our ability to obtain additional funding for operations and to complete the development and commercialization of our drug candidates; and other risks and uncertainties set forth in “Risk Factors” in our most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this press release, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain, and you are cautioned not to rely unduly upon these statements. All information in this press release is as of the date of this press release. The information contained in any website referenced herein is not, and shall not be deemed to be, part of or incorporated into this press release.
i U.S. Food and Drug Administration. (2022, October 14). Final Administrative Order (OTC000027): Internal Analgesic, Antipyretic, and Antirheumatic Drug Products for Over-the-Counter Human Use.
ii ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988; 2:349-60.
iii Rao SV, O’Donoghue ML, Ruel M, et al. 2025 ACC/AHA/ACEP/NAEMSP/SCAI Guideline for the Management of Patients with Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2025; 151:e771-e862.
Aspire Biopharma Holdings, Inc.
Contact
PCG Advisory
Kevin McGrath
+1-646-418-7002
kevin@pcgadvisory.com
SOURCE: Aspire Biopharma Holdings, Inc.

View the original press release on ACCESS Newswire