Secure, scalable, multi-cloud platform using federated learning aims to accelerate cancer discoveries and treatments
SEATTLE, WASHINGTON / ACCESS Newswire / October 1, 2025 / The Cancer AI Alliance (CAIA) – a research collaboration of top cancer centers – today announced the first scalable platform using federated learning for cancer research.
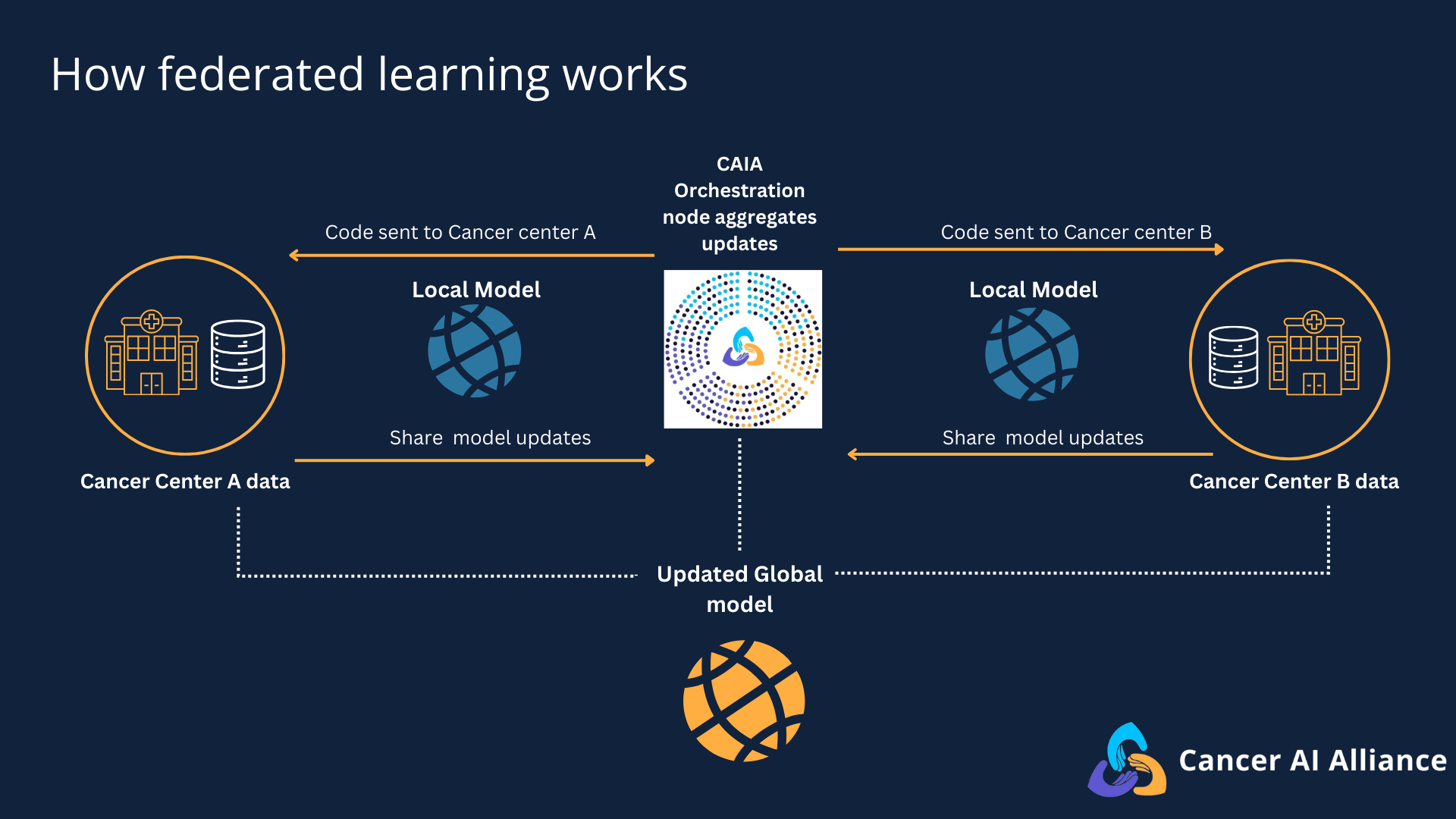
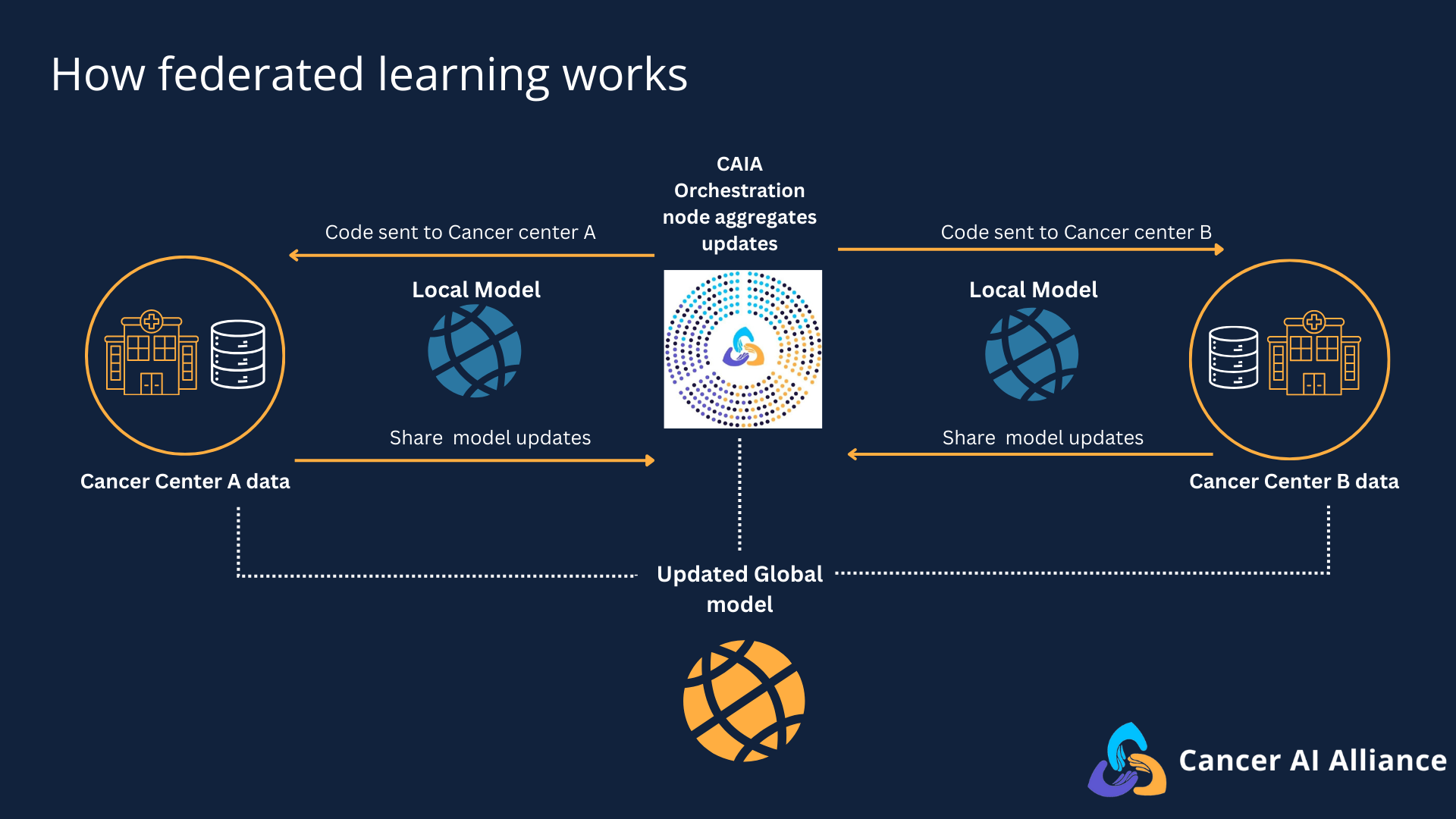
An overview of the federated learning process.
CAIA is comprised of National Cancer Institute-designated cancer centers Dana-Farber Cancer Institute, Fred Hutch Cancer Center, Memorial Sloan Kettering Cancer Center, and The Sidney Kimmel Comprehensive Cancer Center and Whiting School of Engineering at Johns Hopkins with financial and technical support from technology industry leaders Amazon Web Services (AWS), Deloitte, Ai2 (Allen Institute for AI), Google, Microsoft, NVIDIA and Slalom.
The platform serves as the technological foundation of CAIA’s aim to save more lives by enabling researchers and clinicians to train AI models that learn from participating cancer centers’ millions of clinical data points while maintaining data security, privacy and adherence to regulatory and ethical standards.
“We are at an urgent inflection point in both cancer research and AI, and this platform enables us to rise to this moment by rapidly advancing cancer research, treatments and care,” said Jeff Leek, PhD, vice president and chief data officer at Fred Hutch and holder of the J. Orin Edson Foundation Endowed Chair.
“Currently, it takes years to uncover new insights that can be translated into better treatments and care, but this platform will accelerate the pace of breakthrough discoveries by up to tenfold, reducing that time from years to months,” said Leek, who is also the founder and scientific director of CAIA and a member of its steering committee.
“It cannot be overstated how momentous it is that we came together to launch this platform in just one year, and we did it as a unified alliance with a shared mission to eradicate cancer,” said Brian M. Bot, director of the strategic coordinating center for CAIA.
While federated learning – a machine learning method that preserves anonymity of individual data – has been gaining steam for nearly 10 years, adapting the technology for multi-institution use in cancer research has proved elusive due to significant technological, regulatory, patient privacy and data harmonization challenges, as well as the coordination effort necessary to bring together organizations of this scale and complexity.
Development of CAIA’s platform was possible due to an intense focus on collaboration and a drive for collective action across the participating cancer centers and technology companies, resulting in a unified technical, legal and governance structure.
“CAIA represents a strategic shift leveraging collective strength rather than isolation,” said Anaeze Offodile, MD, MPH, chief strategy officer of Memorial Sloan Kettering Cancer Center. “By combining MSK’s clinical expertise with the alliance’s capital, network of technology partners, data and federated framework, we can accelerate meaningful advances in cancer care while upholding the highest standards of security and integrity. Together, we move closer to the shared goal of reducing-and ultimately eliminating-the burden of cancer.”
The federated learning platform works like this: participating cancer centers implement federated learning technology at their institutions, each connecting to a centralized orchestration component of the platform. Using this architecture, AI models travel to each participating cancer center’s secure data to learn from it locally, generating a summary of its learnings without individual clinical data ever leaving institutional firewalls. The insights gained from training the model on each cancer center’s de-identified data are then aggregated centrally to strengthen the AI models to uncover patterns, maximizing the value of the collective knowledge base.
Enabling researchers to develop models on data from multiple cancer centers through a collaborative, federated learning ecosystem creates a paradigm shift from solving problems in isolation to solving them together. More importantly, these updated AI models could significantly improve health outcomes for cancer patients by revealing trends across more diverse populations and rare cancers.
“CAIA will enable unprecedented exploration of AI models for cancer patient data, through a privacy-aware technical framework and a collaborative research alliance,” said Alexis Battle, PhD, professor of biomedical engineering and computer science, and director of the Malone Center for Engineering in Healthcare, and director for Research Strategy and Partnerships, Data Science and AI Institute at the Johns Hopkins Whiting School of Engineering.
“This could serve as a model for every cancer center in the country to join CAIA in this collaborative effort to unlock innovation in cancer care,” continued Vasan Yegnasubramanian, MD, PhD, professor of oncology, pathology, and radiation oncology and molecular radiation sciences at the Johns Hopkins Kimmel Cancer Center and director of inHealth Precision Medicine at Johns Hopkins Medicine. Battle and Yegnasubramanian are members of the steering committee for CAIA.
Eight unique projects are being launched by researchers across the participating cancer centers. These initial projects aim to tackle some of oncology’s most persistent challenges, from predicting treatment response to identifying novel biomarkers and analyzing rare cancer trends. The projects use the federated learning platform and structured, de-identified data housed securely by participating cancer centers, which collectively provides a diverse and representative foundation of over 1 million patients for modeling and analysis.
The platform’s true power, however, lies in its potential to scale up. Over the next year, CAIA plans to enable dozens of research models and add more participants to the alliance.
“With the launch of CAIA we have laid a critical foundation in the effort to accelerate new discoveries and the combined data from our cancer centers can now power these innovative AI models. We are excited to share these models with research centers across the nation and exponentially expand access to the data that will drive progress toward better diagnosis, treatment and outcomes for cancer patients everywhere,” said Eliezer Van Allen, MD, Chandra Nohria Family Chair for AI in Cancer Research at Dana-Farber Cancer Institute.
# # #
Additional CAIA collaborator quotes
“AWS is immensely proud to help power this industry-first collaboration with our cloud technology and LLM platforms, in support of CAIA’s mission to transform cancer research and care,” said Dr. Angela Shippy, Senior Physician Executive and Clinical Innovation Lead at Amazon Web Services. “Collaboration often leads to discovery, and I’m eager to see what medical breakthroughs come from the collective expertise of these world-class cancer centers and global technology leaders.”
“At Deloitte, we believe collaboration drives progress,” said Jason Girzadas, CEO of Deloitte US. “CAIA brings together leaders across industries to address one of the world’s most critical health challenges. The unprecedented speed of developing and delivering CAIA’s platform is made possible through each individual group driving toward this common goal, and Deloitte is proud to bring AI and strategy expertise to the Alliance.”
“We are proud to be part of this effort to bring the power of AI to the complex problem of cancer research,” said Ali Farhadi, CEO of Ai2. “CAIA’s ability to bring together cancer centers in a collaborative effort with our state of the art AI tools is a powerful combination that will accelerate scientific progress and save lives. It’s been incredible to see our tools in the hands of oncologists and researchers, where their early efforts have already demonstrated incredible potential to unlock discoveries that could reshape healthcare and deliver life-changing outcomes for patients.”
“Google is honored to continue to partner with the Cancer AI Alliance,” said Reymund Dumlao, Director, State and Local Government and Education, Google Public Sector. “This groundbreaking initiative represents a pivotal moment in our collective pursuit to eradicate cancer. By integrating advanced federated AI technologies with the expertise and data resources of leading cancer centers, we are establishing a secure, scalable platform designed to accelerate scientific discovery. This collaborative ecosystem will enable a unified effort against cancer, transforming research methods and expediting the delivery of innovative treatments to patients globally.”
“The collaborative work that CAIA has done will revolutionize how medical research is done, accelerate scientific discovery, and save lives,” said Dr. William Weeks, Director of Health, Microsoft AI for Good Lab.
“At Slalom, we see the future of health as being the convergence of technology, data, and strategy to build better tomorrows for all,” said Brenda Young, managing director at Slalom. “CAIA demonstrates how collaboration between leading cancer centers and technology partners can unlock new insights and reshape the use of Data & AI to diagnose, treat, and prevent cancer. We look forward to our continued partnership as we pave the way for transformative breakthroughs in health.”
Media Contact:
Molly McElroy and Christina VerHeul
media@fredhutch.org
206-667-2210
About the Cancer AI Alliance (CAIA)
The Cancer AI Alliance (CAIA) is comprised of the nation’s top cancer centers with support from technology industry leaders. CAIA aims to accelerate cancer discovery and treatment. Since its founding in 2024, CAIA has secured $65M in financial and technical support. Through this unprecedented collaboration, CAIA launched the first scalable, multi-cloud federated learning platform for cancer research, which enables researchers to train AI models on diverse, multi-institutional clinical data while maintaining data security and privacy. By combining the collective knowledge of its members, CAIA is on a mission to redefine the cancer research landscape.
Dana-Farber Cancer Institute
Dana-Farber Cancer Institute is one of the world’s leading centers of cancer research and treatment. Dana-Farber’s mission is to reduce the burden of cancer through scientific inquiry, clinical care, education, community engagement and advocacy. Dana-Farber is a federally designated Comprehensive Cancer Center and a teaching affiliate of Harvard Medical School.
Dana-Farber is the only hospital nationwide with a top 3 U.S. News & World Report Best Cancer Hospital ranking in both adult and pediatric care.
As a global leader in oncology, Dana-Farber is dedicated to a unique and equal balance between cancer research and care, translating the results of discovery into new treatments for patients locally and around the world, offering more than 1,100 clinical trials.
Fred Hutch Cancer Center
Fred Hutch Cancer Center unites individualized care and advanced research to provide the latest cancer treatment options while accelerating discoveries that prevent, treat and cure cancer and infectious diseases worldwide.
Based in Seattle, Fred Hutch is an independent, nonprofit organization and the only National Cancer Institute-designated cancer center in Washington. We have earned a global reputation for our track record of discoveries in cancer, infectious disease and basic research, including important advances in bone marrow transplantation, immunotherapy, HIV/AIDS prevention and COVID-19 vaccines. Fred Hutch operates eight clinical care sites that provide medical oncology, infusion, radiation, proton therapy and related services. Fred Hutch also serves as UW Medicine’s cancer program.
Johns Hopkins University
As the nation’s first research university, Johns Hopkins’ mission is to foster cutting-edge research and innovation, to educate its students and cultivate their capacity for lifelong learning, and to bring thebenefits of discovery to the world through its 10 world-renowned schools, and numerous institutes and centers.
Sidney Kimmel Comprehensive Cancer Center
Since our founding more than 50 years ago, our medical pioneers led the way to understanding cancer through impactful research, developing novel therapies, and attending to the complex needs of patients. Our experts mapped the genomic blueprints for cancer, deciphered the epigenome, discovered ways to make the immune system fight cancer, eliminated the need for perfect matches in bone marrow transplant, pioneered life-saving surgeries, and brought proton therapy and other advanced technologies in radiation therapy.
Whiting School of Engineering
We advance data- and science-driven engineering discovery, innovation, and systems applications to help everyone live longer and healthier lives and empower communities and society to thrive even in extreme environments. With more than 26 research centers and institutes – including the Data Science and AI Institute – and through strong community, university, and industry partnerships, Hopkins Engineering is a driving force in the future of technology, health care, and engineering education.
Memorial Sloan Kettering Cancer Center
The people of Memorial Sloan Kettering Cancer Center (MSK) are united by a singular mission: ending cancer for life. Our specialized care teams provide personalized, compassionate, expert care to patients of all ages. Informed by basic research done at our Sloan Kettering Institute, scientists across MSK collaborate to conduct innovative translational and clinical research that is driving a revolution in our understanding of cancer as a disease and improving the ability to prevent, diagnose, and treat it. MSK is dedicated to training the next generation of scientists and clinicians, who go on to pursue our mission at MSK and around the globe. One of the world’s most respected comprehensive centers devoted exclusively to cancer, we have been recognized as one of the top two cancer hospitals in the country by U.S. News & World Report for more than 30 years. www.mskcc.org
Contact Information
Christina VerHeul
Associate Vice President, Communications
media@fredhutch.org
(206) 667-2210
Molly McElroy
Sr. Communications Manager
mwmcelro@fredhutch.org
206-667-2210
SOURCE: Fred Hutch Cancer Center
View the original press release on ACCESS Newswire